This article was released as Pharm Edaily Premium Content on November 12, 2025, at 6:05 PM.
[Kim Saemi, Edaily Reporter] On the 12th, ABL Bio led a general market rally as it secured a major deal worth 3.8 trillion KRW with Eli Lilly. The biotech sector saw strong demand, with rising interest in companies like Sinteca Bio, which is benefitting from government support. Meanwhile, Genome & Company plans to focus on antibody-drug conjugate (ADC) development after announcing results of a domestic Phase 2 trial for its microbiome-based cancer immunotherapy, GEN-001.
ABL Bio’s 3.8 Trillion KRW ‘Big Deal’… What’s Next for Tech Exports?
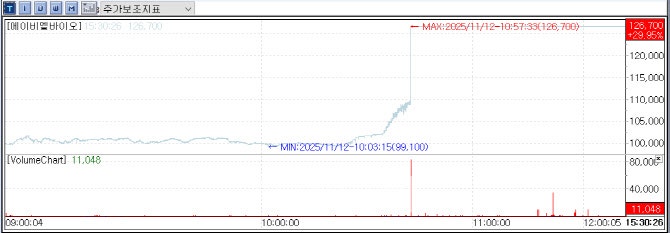
According to KG Zeroin MP Doctor(formerly MarketPoint), ABL Bio’s stock surged by 29.95%, closing at 126,700 KRW, a rise of 29,200 KRW compared to the previous day.
|
The technology transfer news attracted attention to the biotech sector, with companies such as Alteogen, LigaChem Biosciences, Olix Pharmaceuticals, and Peptaron being seen as potential candidates for future tech exports.
Alteogen, the top KOSDAQ market cap company, has mentioned that one or two technology transfer deals could be expected by year-end. Investors are speculating that deals involving bispecific antibodies or ADC technologies might be announced soon. Alteogen’s stock rose by 8.04%, reaching a new 52-week high at 551,000 KRW.
Interest in LigaChem Biosciences, known as a leader in technology exports, also increased, with the stock soaring by 17.56%, closing at 170,700 KRW, also a new 52-week high. Olix Pharmaceuticals, which had been selected by Eli Lilly for a partnership, also surged by 15.16%, closing at 139,000 KRW, setting a new 52-week high.
Given the size of the deal with Eli Lilly, market attention also shifted to Peptaron, which signed a technology evaluation contract with Eli Lilly in October last year. This evaluation is expected to conclude by December, and investors had hoped for a tech export deal by year-end. Delays into next year have caused fluctuations in the stock. However, today, Peptron’s stock rose by 28,000 KRW (10.53%), closing at 294,000 KRW, as expectations of a technology transfer were once again reflected in the market.
Sinteca Bio Hits Upper Limit as AI Drug Stocks Surge on Government Support
At the beginning of trading, Sinteca Bio saw its stock hit the upper limit, boosting the AI-driven drug development sector. By 9:04 AM, Sinteca Bio’s stock had surged by 29.77%, reaching 5,470 KRW, and maintained its position at the upper limit for the rest of the day. AI drug developer OncoCross also rose by 16.76%, closing at 12,330 KRW.
|
AI drug developers have been seeing volatile stock movements recently. On the 11th, Pharose Eye Bio surged to the upper limit after its drug candidate for acute myeloid leukemia (AML) was listed by the World Health Organization (WHO).
The strong performance of AI drug developers is seen as a reflection of the market’s expectations that government support will translate into tangible results. Recently, several key government agencies, including the Ministry of Health and Welfare, the Ministry of Science and ICT, and the Ministry of SMEs and Startups, have launched AI-focused drug development projects.
The Ministry of Health and Welfare announced plans to launch the K-AI drug development R&D project on the 5th, which is expected to rapidly advance domestic AI drug candidates into clinical stages through large-scale national projects. The Ministry of Science and ICT is investing 18.2 billion KRW to launch an AI-specialized foundation model project from this month through September next year, with participation from companies such as Lunit and SK Biopharm.
A representative from Sinteca Bio commented, “The overall rise in AI drug development stocks seems to be benefiting from sector-wide demand.”
Genome & Company Shifts Focus to ADC After Microbiome Development
At 1:22 PM, Genome & Company released the results of its Phase 2 clinical trial for GEN-001, a treatment for stomach cancer, in Korea. Following the announcement, the company’s stock rose by 18.44%, reaching a high of 3,494 KRW, surpassing the 52-week trading volume record.
|
While the results indicated some positive responses, particularly in terms of response durability among a small subset of patients, the data did not show significant benefits in terms of OS or PFS.
Despite these mixed results, the company’s stock ended the day 9.83% higher, closing at 3,240 KRW.
Genome & Company has stated that it does not plan to conduct additional clinical trials for GEN-001 but may continue to develop it with overseas partners. The company’s shift in focus towards ADC technology is expected to limit the impact of the GEN-001 trial results on its overall valuation. A company representative noted, “Genome & Company does not plan to continue further clinical trials for GEN-001, but if we find overseas partners, we could continue development.”
댓글목록
등록된 댓글이 없습니다.